You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Alcohol ABV drop after some time
- Thread starter Laudurgs
- Start date

Help Support Homebrew Talk - Beer, Wine, Mead, & Cider Brewing Discussion Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Laudurgs
Member
- Joined
- Jan 27, 2022
- Messages
- 5
- Reaction score
- 0
Laudurgs
Member
- Joined
- Jan 27, 2022
- Messages
- 5
- Reaction score
- 0
Dunno, afterwards I squeeze all the peels through juice press, but total amount doesnt change
Laudurgs
Member
- Joined
- Jan 27, 2022
- Messages
- 5
- Reaction score
- 0
If addin sugar, then no, they dont show accurate readings. IIRC sugar may be the answer of lower reading, therefor Im waiting test results from official lab.Does the alcohol hydrometer work with solutions that have lots of lemon extracts in them? IIRC sugars will throw it off. That's a lot of lemon you added.
600 lemons in 20 litres
The alcoholmeter that you use to measure ABV is just a simple hydrometer, and only reacts to the solution density, not the specific components of the solution. Alcohol has a density significantly lower than water, so mixtures of alcohol and water also have a lower density than water. The alcoholmeter only measures ABV accurately when the solution contains only ethanol and water.
Soaking the lemon zest in the high ABV solution caused other things, which have densities higher than water, to go into solution and thus raise the density of the solution. The alcoholmeter then floats higher in the solution, and the number you read is lower. This doesn't mean the ABV changed significantly, but rather it means that the alcoholmeter is no longer reading correctly, because the solution now contains more than just ethanol and water.
Brew on
Soaking the lemon zest in the high ABV solution caused other things, which have densities higher than water, to go into solution and thus raise the density of the solution. The alcoholmeter then floats higher in the solution, and the number you read is lower. This doesn't mean the ABV changed significantly, but rather it means that the alcoholmeter is no longer reading correctly, because the solution now contains more than just ethanol and water.
Brew on

this is yet another case where i think a refractometer reading would be handy.....to compare the two....
Be even harder to figure out what the refractometer reading meant. Having both would totally confuse you.this is yet another case where i think a refractometer reading would be handy.....to compare the two....
Brew on

Be even harder to figure out what the refractometer reading meant. Having both would totally confuse you.
Brew on
and yet i can get a good idea of a refrac reading on 93% ABV....
so if a proof and trales hydro is reading 75% (0.84 SG) a refrac would read 25 BRIX if it's actually 93% ABV?

and if it did lose ABV somehow, then the refrac would be like 15 BRIX?
No. Once you get into three (or more) constituent solutions, any instrument calibrated based on a measured parameter vs. binary solution parameter correlation becomes useless (unless the extra component(s) behave substantially similar to one of the binary constituents.)and yet i can get a good idea of a refrac reading on 93% ABV....
so if a proof and trales hydro is reading 75% (0.84 SG) a refrac would read 25 BRIX if it's actually 93% ABV?
and if it did lose ABV somehow, then the refrac would be like 15 BRIX?
Brew on

No. Once you get into three (or more) constituent solutions, any instrument calibrated based on a measured parameter vs. binary solution parameter correlation becomes useless (unless the extra component(s) behave substantially similar to one of the binary constituents.)
Brew on
i was wondering if something like pectin would throw it off, from the assumtion, sugar/water/ethanol.....but i would say it works flawless so far for me....
and i still think it's be a good guess if somehow the OP lost 20% ABV....

just in case you so chose to try my idea, remember this is weight though...and a proof hydro is ABV...so multiply the hydro reading by 0.88
i still think it'd be revealing to do.....i just tried it with my brandy that's been aging on oak for 13 years...got a brix of 19, and a proof hydro reading of 57%.....so it seems to work even with wood sugars present?
Laudurgs
Member
- Joined
- Jan 27, 2022
- Messages
- 5
- Reaction score
- 0
Just got back lab results, +/- 0.5% same reading as in home with alcoholmeterThe alcoholmeter that you use to measure ABV is just a simple hydrometer, and only reacts to the solution density, not the specific components of the solution. Alcohol has a density significantly lower than water, so mixtures of alcohol and water also have a lower density than water. The alcoholmeter only measures ABV accurately when the solution contains only ethanol and water.
Soaking the lemon zest in the high ABV solution caused other things, which have densities higher than water, to go into solution and thus raise the density of the solution. The alcoholmeter then floats higher in the solution, and the number you read is lower. This doesn't mean the ABV changed significantly, but rather it means that the alcoholmeter is no longer reading correctly, because the solution now contains more than just ethanol and water.
Brew on
No, you don't convert Wt% ethanol to Vol% (ABV) ethanol by multiplying by 0.88, nor vice versa. The relationship is non-linear because ethanol/water solutions do not behave like ideal solutions (the volume of the solution is less then the sum of the volumes of the individual components.) The table gives you the information you need to convert Wt% to Vol%. Can you figure out the formula.View attachment 757823
just in case you so chose to try my idea, remember this is weight though...and a proof hydro is ABV...so multiply the hydro reading by 0.88
i still think it'd be revealing to do.....i just tried it with my brandy that's been aging on oak for 13 years...got a brix of 19, and a proof hydro reading of 57%.....so it seems to work even with wood sugars present?
The Brix scale is for solutions of sucrose in water, not alcohol and water. They do make refractometers for for ethanol/water solutions, but they do not read Brix (the ethanol/water scale is much more non-linear that the sucrose/water scale) It would be possible to correlate the ABV reading of an ethanol/water refract to the Brix scale of a sucrose/water refract. Have you done this? And if not, how are you correlating Brix to ABV?
Compare the scale from the ethanol/water refractometer below, to the one you are familiar with in a Brix refractometer. Notice how the scale compresses rapidly as you approach 100% ABV. This is the opposite of the SG scale which expands as you approach 100%ABV. The the refract gets less accurate at high ABV, whereas the hydrometer gets more accurate at high ABV.
Oak aging shouldn't change the measured ABV of a distilled spirit significantly, whether measured by an (appropriate) refract or a hydrometer, as the amount of material actually dissolved from the oak is very small. Barrel aging for long periods might change the ABV depending on whether the angels drink a different ratio of alcohol & water than the original spirit contained.
Brew on

What method did the lab use for measuring ABV?Just got back lab results, +/- 0.5% same reading as in home with alcoholmeter
Brew on

No, you don't convert Wt% ethanol to Vol% (ABV) ethanol by multiplying by 0.88, nor vice versa. The relationship is non-linear because ethanol/water solutions do not behave like ideal solutions (the volume of the solution is less then the sum of the volumes of the individual components.) The table gives you the information you need to convert Wt% to Vol%. Can you figure out the formula.
The Brix scale is for solutions of sucrose in water, not alcohol and water. They do make refractometers for for ethanol/water solutions, but they do not read Brix (the ethanol/water scale is much more non-linear that the sucrose/water scale) It would be possible to correlate the ABV reading of an ethanol/water refract to the Brix scale of a sucrose/water refract. Have you done this? And if not, how are you correlating Brix to ABV?
Compare the scale from the ethanol/water refractometer below, to the one you are familiar with in a Brix refractometer. Notice how the scale compresses rapidly as you approach 100% ABV. This is the opposite of the SG scale which expands as you approach 100%ABV. The the refract gets less accurate at high ABV, whereas the hydrometer gets more accurate at high ABV.
Oak aging shouldn't change the measured ABV of a distilled spirit significantly, whether measured by an (appropriate) refract or a hydrometer, as the amount of material actually dissolved from the oak is very small. Barrel aging for long periods might change the ABV depending on whether the angels drink a different ratio of alcohol & water than the original spirit contained.
Brew on
well man, being i didn't take a FG reading on this last batch of beer....not really anyway till it was kegged...because of pressure fermenting....
i did take an OG with a hydro.. ~1.056, added 1lb table sugar, but a bit more on the scale....and after two weeks i get a BRIX of 5.8, and a hydro reading of .999
which every calculator that corrects BRIX with alcohol for the pressence of alcohol...it works to tell me the OG and ABV, if i tell IT the OG it works to tell me the SG?
lol it just works for me?

edit: i'd imagine what ever the calcs i use are using is like solving a quadratic equation?
Last edited:
Using a BRIX refract to calculate an estimate for FG (if you know the OG) for beer is totally different than using a refract on distilled spirits. The formulas for FG are just curve fit polynomials (usually 2nd order) that correlate hydrometer measurements to refract measurements on beer. I also find good agreement between refract estimated FGs and hydro measured FGs.well man, being i didn't take a FG reading on this last batch of beer....not really anyway till it was kegged...because of pressure fermenting....
i did take an OG with a hydro.. ~1.056, added 1lb table sugar, but a bit more on the scale....and after two weeks i get a BRIX of 5.8, and a hydro reading of .999
which every calculator that corrects BRIX with alcohol for the pressence of alcohol...it works to tell me the OG and ABV, if i tell IT the OG it works to tell me the SG?
lol it just works for me?
edit: i'd imagine what ever the calcs i use are using is like solving a quadratic equation?
Brew on

- Joined
- May 28, 2018
- Messages
- 3,048
- Reaction score
- 2,474
If not measurement issue, is it possible it is diluted as it is mixed/absorbed with lemon?
One other possibility, if a long time has past, is the plastic is not completely impermeable. A lot of plastic containers breath more than one might think, any loss of volume?
One other possibility, if a long time has past, is the plastic is not completely impermeable. A lot of plastic containers breath more than one might think, any loss of volume?
Using a BRIX refract to calculate an estimate for FG (if you know the OG) for beer is totally different than using a refract on distilled spirits. The formulas for FG are just curve fit polynomials (usually 2nd order) that correlate hydrometer measurements to refract measurements on beer. I also find good agreement between refract estimated FGs and hydro measured FGs.
Brew on
ok, but when i measure the SG AND BRIX i can tell what the OG was and ABV? or you're very right with distilled spirits, JUST ABV....because when i tested my hooch, it gave me an OG estimate of like 1.330 or something.....but it was spot on for the ABV....
and man i'm not trying to be a dick....just argumentative to try and figure this out? so far it works good....and i wish i knew it did back when i was trying to figure out how much ABV my attempts at making sake had....
this is what i'm using...and you're telling it's bogus? because so far it works great for different brands of apple juice i add sugar to and don't stir in and take a gravity reading first?
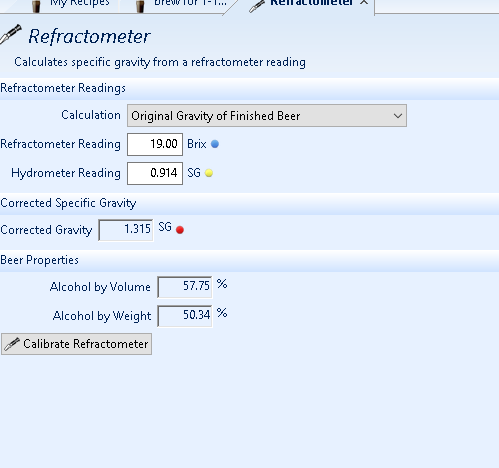
and that prediction for the ABV on that booze is exactly what the proof hydro said? and that's what both my analog & digital refrac told me?
Last edited:
Back calculating OG from hydro and Brix readings of FG is just using the reverse correlation used to find FG from OG and an end of fermentation Brix reading. You use the same data to fit a polynomial, just reverse the independent and dependent variables.ok, but when i measure the SG AND BRIX i can tell what the OG was and ABV? or you're very right with distilled spirits, JUST ABV....because when i tested my hooch, it gave me an OG estimate of like 1.330 or something.....but it was spot on for the ABV....
and man i'm not trying to be a dick....just argumentative to try and figure this out? so far it works good....and i wish i knew it did back when i was trying to figure out how much ABV my attempts at making sake had....
this is what i'm using...and you're telling it's bogus? because so far it works great for different brands of apple juice i add sugar to and don't stir in and take a gravity reading first?
View attachment 757893
and that prediction for the ABV on that booze is exactly what the proof hydro said? and that's what both my analog & digital refrac told me?
Brew on

OP said volume of liquid was the same before and after. If a solution of 94% alcohol dropped to 75%, then 1 L started out with 0.94 L of Eth and 0.06 L of water, and finished at 0.06 L / 0.25 = 0.24 L total, for a volume loss of 0.76 L. You would definitely notice a loss of 3/4 of your starting volume! (The preceding is not totally accurate because it assumes ethanol and water form ideal solutions, which we know is not the case.)If not measurement issue, is it possible it is diluted as it is mixed/absorbed with lemon?
One other possibility, if a long time has past, is the plastic is not completely impermeable. A lot of plastic containers breath more than one might think, any loss of volume?
On the other hand, if you added water to the original 1 L of 94% Eth to reach 75%, the volume would increase to 0.94/0.75 = 1.25 L. (Again non-linearity is ignored.)
Brew on

OP said volume of liquid was the same before and after. If a solution of 94% alcohol dropped to 75%, then 1 L started out with 0.94 L of Eth and 0.06 L of water, and finished at 0.06 L / 0.25 = 0.24 L total, for a volume loss of 0.76 L. You would definitely notice a loss of 3/4 of your starting volume! (The preceding is not totally accurate because it assumes ethanol and water form ideal solutions, which we know is not the case.)
On the other hand, if you added water to the original 1 L of 94% Eth to reach 75%, the volume would increase to 0.94/0.75 = 1.25 L. (Again non-linearity is ignored.)
Brew on
The tub was pretty full of lemon rind, that had to be where the water came from; then when the lemon was removed, the rind carried off the excess volume?
Do you have any idea what the original liquid volume was, and what the volume was after combining the lemon zest and liquid?The tub was pretty full of lemon rind, that had to be where the water came from; then when the lemon was removed, the rind carried off the excess volume?
Brew on

Similar threads
- Replies
- 104
- Views
- 5K
- Replies
- 7
- Views
- 809


