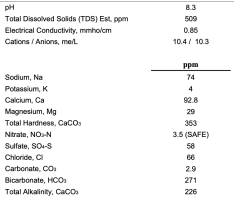
I've brewed with fairly hard water over the years with moderate levels of sodium and sulfate. I just moved to a new area and the water appears to be much harder to me, with high levels of sodium (74ppm) and sulfate (174ppm, converted)---see the attached image.
In part this may work well with darker beers (minus the high sulfate) but wondering how to approach beers that lean towards yellow. In the past, with high hardness and bicarbonate, I've always diluted 50/50 with the previous water I've brewed with if I was making something like an IPA, saison or kolsch, but it appears that I may have to dilute more with this water.
Am I just better off building from distilled when brewing lighter beers? If so, is there anything I need to take into account beyond just hitting the targeted Ca, Mg, Na, SO4 and Cl?
In part this may work well with darker beers (minus the high sulfate) but wondering how to approach beers that lean towards yellow. In the past, with high hardness and bicarbonate, I've always diluted 50/50 with the previous water I've brewed with if I was making something like an IPA, saison or kolsch, but it appears that I may have to dilute more with this water.
Am I just better off building from distilled when brewing lighter beers? If so, is there anything I need to take into account beyond just hitting the targeted Ca, Mg, Na, SO4 and Cl?