FunkedOut
FunkedOver
- Joined
- Mar 23, 2017
- Messages
- 850
- Reaction score
- 418
Looking for confirmation of my thought from those smarter than I.
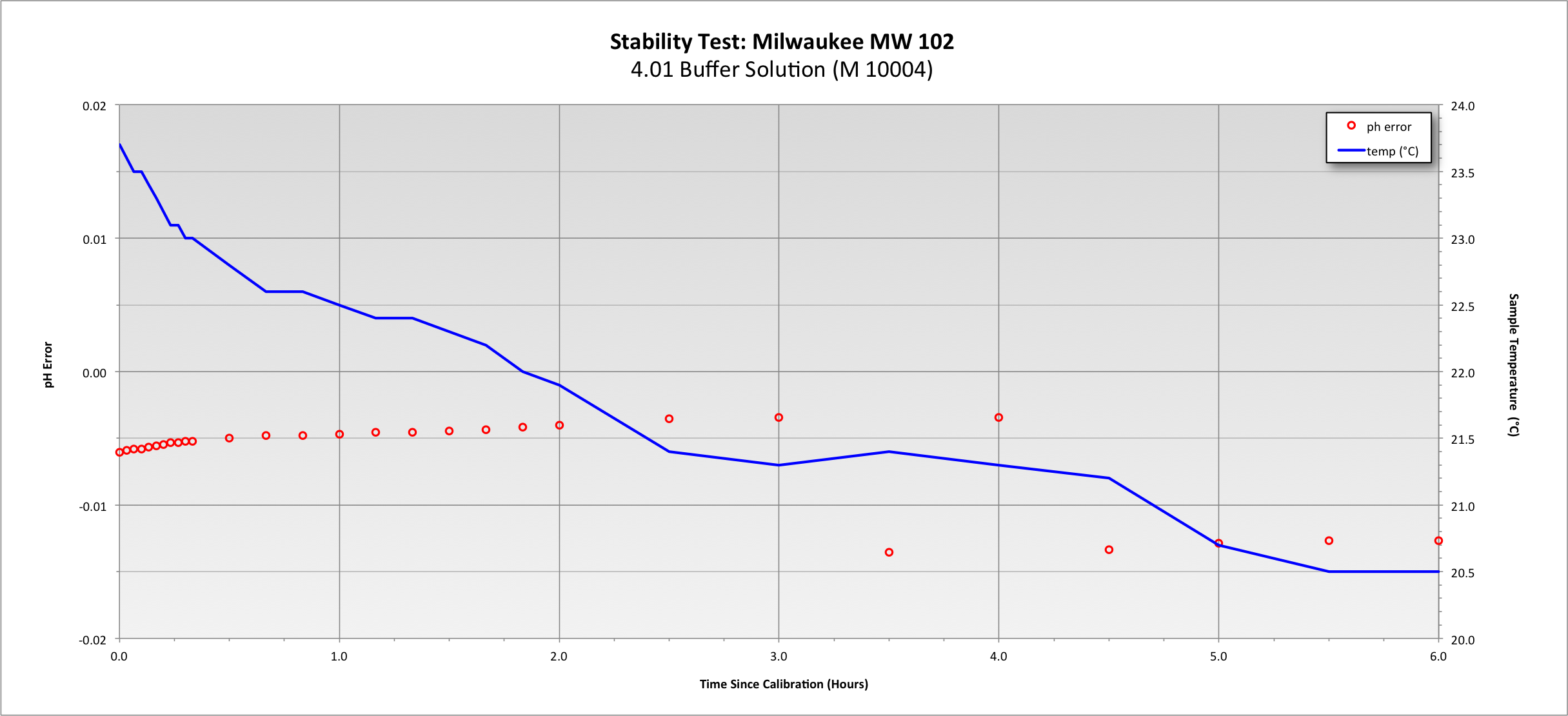
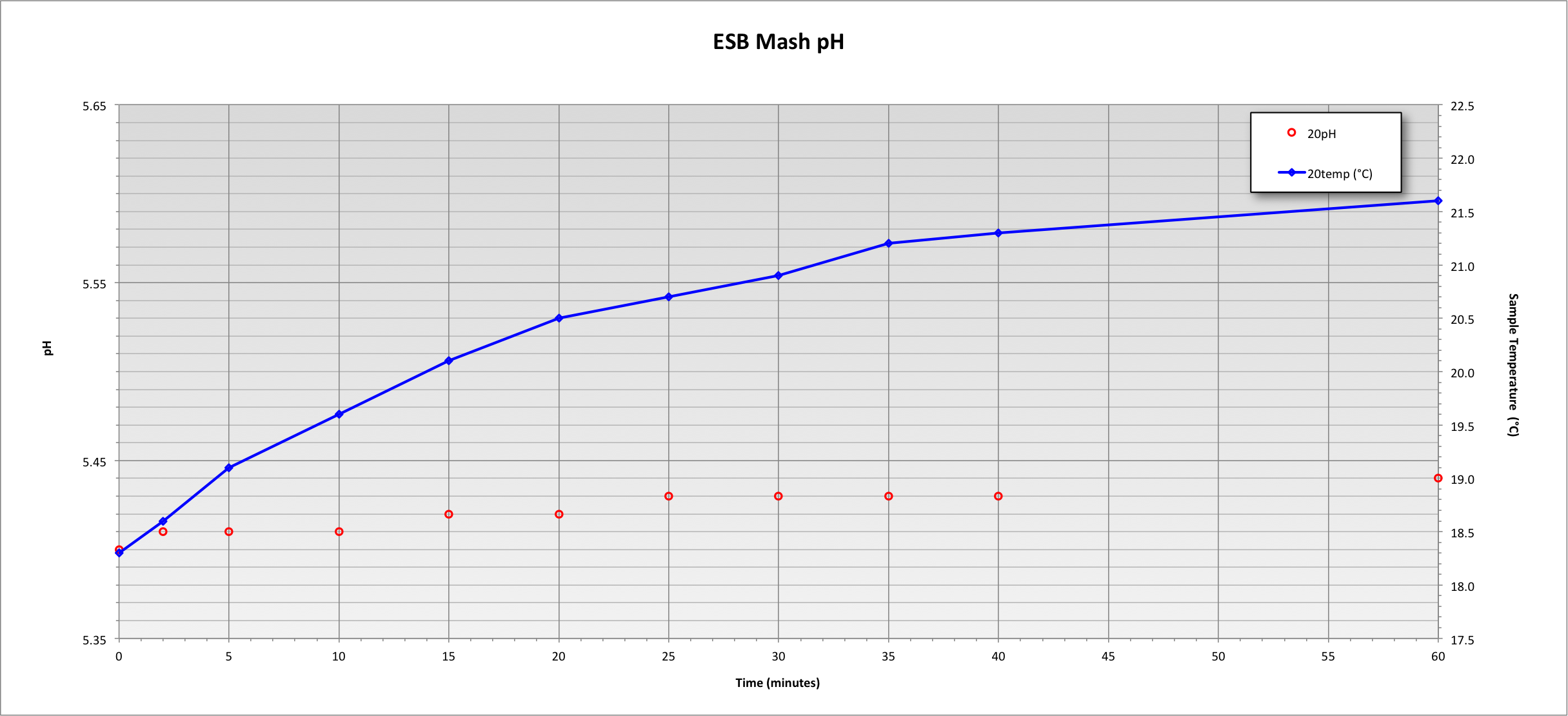
I always let my mash sample sit for hours before I measure the ph.
Does this time allow for the mash sample ph to drift/change?
Or is this fine?
More details about my process:
Underlet my mash. Usually takes ~5 minutes to get ~5 gallons in.
Stir the mash well.
Cover and let sit for 20 minutes.
Draw ~2 quarts off the drain valve and pour back in the top.
(Mash tun has zero dead space and 1.5 quarts below the false bottom)
Draw my small sample (~2oz) and place in the freezer.
When the mash is done, I pull the ph sample out of the freezer and place at room temperature.
Sometimes, I'll make to back-to-back batches this way.
At the end of the day, after pitching and cleaning, I'll clean myself up and gather notes including measuring these samples ph.
For right now, I'm not adjusting the ph on the fly, as my measurements are close enough.
I'm just gathering data for future brews.
My question is more about the accuracy of my measurements.
Do these few hours of sitting around at room temp change the ph in any way?
I think not, but I know not.
Thanks in advance.
I always let my mash sample sit for hours before I measure the ph.
Does this time allow for the mash sample ph to drift/change?
Or is this fine?
More details about my process:
Underlet my mash. Usually takes ~5 minutes to get ~5 gallons in.
Stir the mash well.
Cover and let sit for 20 minutes.
Draw ~2 quarts off the drain valve and pour back in the top.
(Mash tun has zero dead space and 1.5 quarts below the false bottom)
Draw my small sample (~2oz) and place in the freezer.
When the mash is done, I pull the ph sample out of the freezer and place at room temperature.
Sometimes, I'll make to back-to-back batches this way.
At the end of the day, after pitching and cleaning, I'll clean myself up and gather notes including measuring these samples ph.
For right now, I'm not adjusting the ph on the fly, as my measurements are close enough.
I'm just gathering data for future brews.
My question is more about the accuracy of my measurements.
Do these few hours of sitting around at room temp change the ph in any way?
I think not, but I know not.
Thanks in advance.