Okay , so I'm using Bru N Water . For my 5.5 gallon IPA I'm using the following.
11.5 # grain bill
4.6 gallon mash - 3 gallons sparge
Gypsum
Mash = 5.9g
Sparge = 2.8g
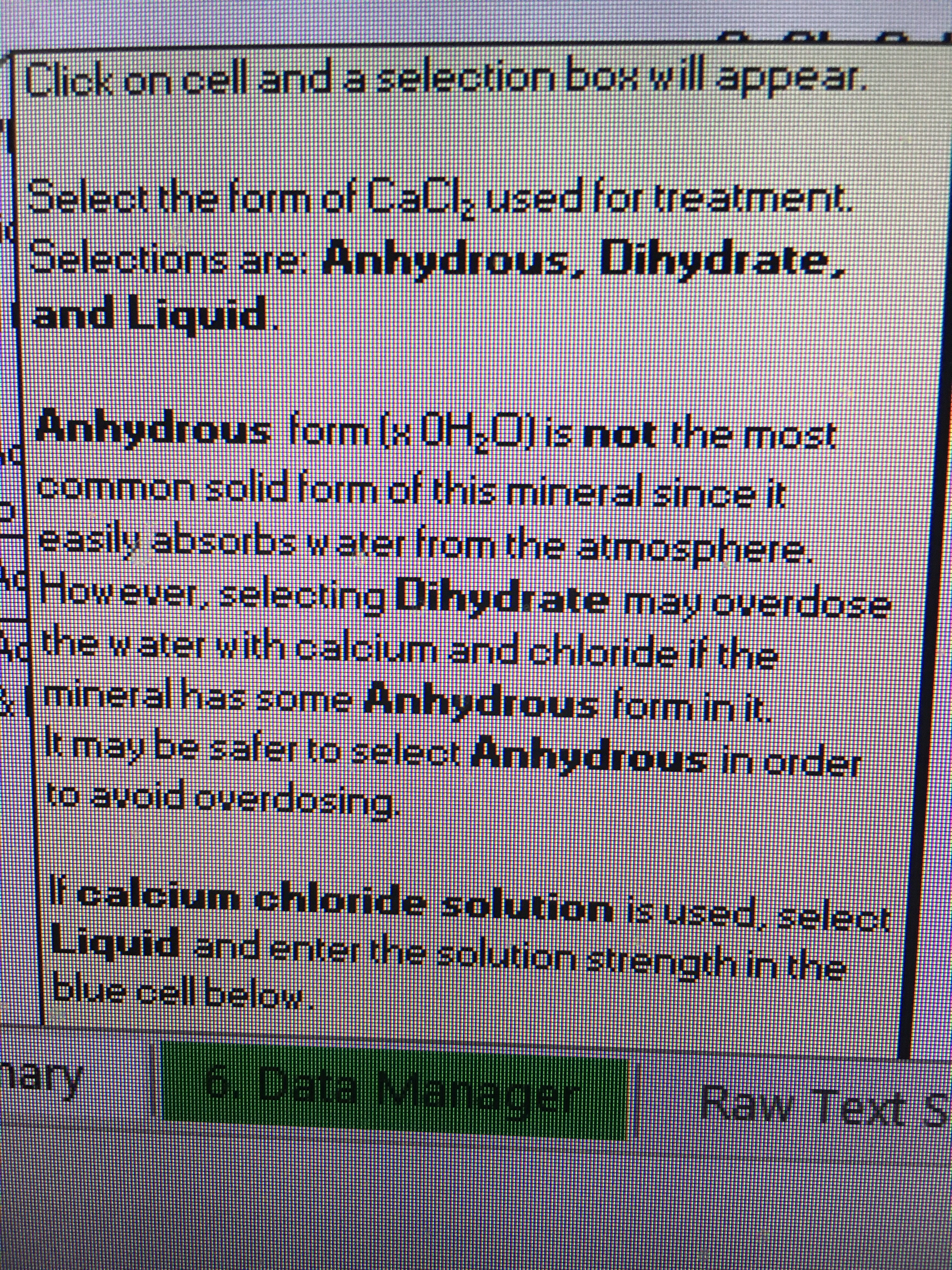
CaCI
Mash = .5g
Sparge = .3g
Lactic Acid
Mash = 2.7ml
Sparge = 1ml
I get roughly 5.28 - 5.30 ph
Now I'm scaling my brew up to 66 gallons . The Gypsum comes out in this batch to 62g in mash and 60g in sparge . CaCI is 16g in mash and 15.8 in sparge . Lactic Acid 9.2ml in mash and 2.0ml in sparge . Giving me a mash ph of 5.3
The Gypsum is the only thing that bumped up somewhat linear. Is there something I'm missing ?
11.5 # grain bill
4.6 gallon mash - 3 gallons sparge
Gypsum
Mash = 5.9g
Sparge = 2.8g
CaCI
Mash = .5g
Sparge = .3g
Lactic Acid
Mash = 2.7ml
Sparge = 1ml
I get roughly 5.28 - 5.30 ph
Now I'm scaling my brew up to 66 gallons . The Gypsum comes out in this batch to 62g in mash and 60g in sparge . CaCI is 16g in mash and 15.8 in sparge . Lactic Acid 9.2ml in mash and 2.0ml in sparge . Giving me a mash ph of 5.3
The Gypsum is the only thing that bumped up somewhat linear. Is there something I'm missing ?